PROTONS AND NEUTRONS MAKE UP THE MASS OF THE ATOM BECAUSE THEY EACH HAVE A MASS OF 1 AMU. This is an integer for a given nucleusisotope.

What Is An Atomic Number Definition And Examples
What are the mass numbers for the following atoms.
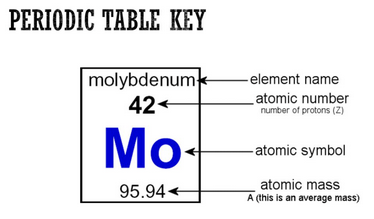
Why is the number called a mass number. Another name for mass number is also called the atomic mass number. Why is this number called a mass number. Isotopes of elements are distinguished by their mass number.
- the answers to brainsanswerscouk. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. For example carbon-12 and carbon-14 have mass numbers of 12 and 14 respectively.
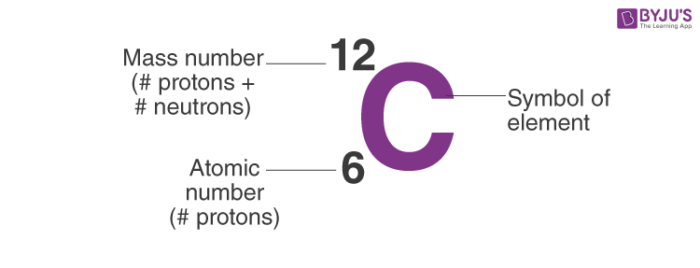
Mass number in nuclear physics the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number is commonly cited in distinguishing among the isotopes of an element all of which have the same atomic number number of protons and are represented by the same. Atomic number proton number plus neutron number equals mass number.
The number of protons and neutrons added together. It is a decimal number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.
3 on a question B. Add your answer and earn points. In other words it is the sum of the number of nucleons in an atom.
B How is the mass number of an isotope. These are the only subatomic particles that have substantial mass. The mass number is commonly cited in distinguishing.
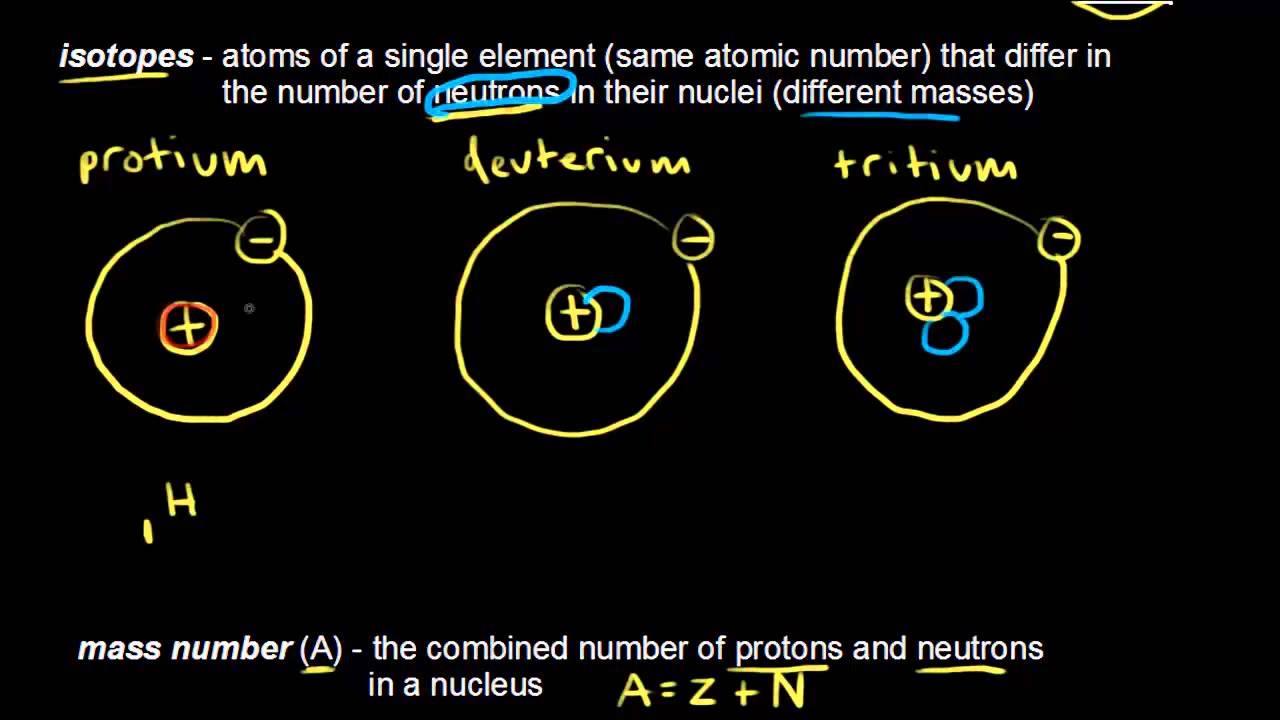
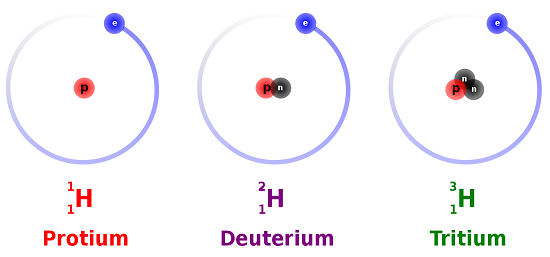
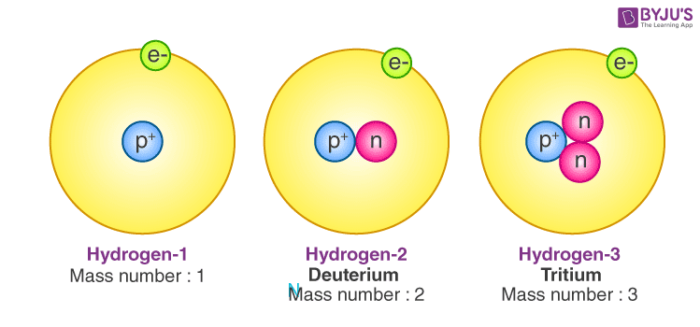
It indicates the integer sum of the nucleons that is the nuclear particles - protons and neutrons. The mass number is the sum of the number of protons and neutrons in an atom. The A stands for the German word atomgewichte translated as atoms weight it even sounds a bit like it in English.
The mass number also called nucleon number refers to the total number of protons and neutrons in the nucleus of an atom and is used to organize the chart of nuclides. While atomic mass is an absolute mass relative isotopic mass is a dimensionless number with no units. It is also called atomic mass number or nucleon number.
An elements mass number A is the sum of the number of protons and the number of neutrons. Atomic mass is different from mass number and it is a fraction since it is the average atomic mass of all the isotopes of an atom. Why is mass number called A.
Atom I Atom II Number of Protons 5 9 Number of Neutrons 6 7 Mass Number. Why is this number called a mass number. Mass number is an integer whole number equal to the sum of the number of protons and neutrons of an atomic nucleus.
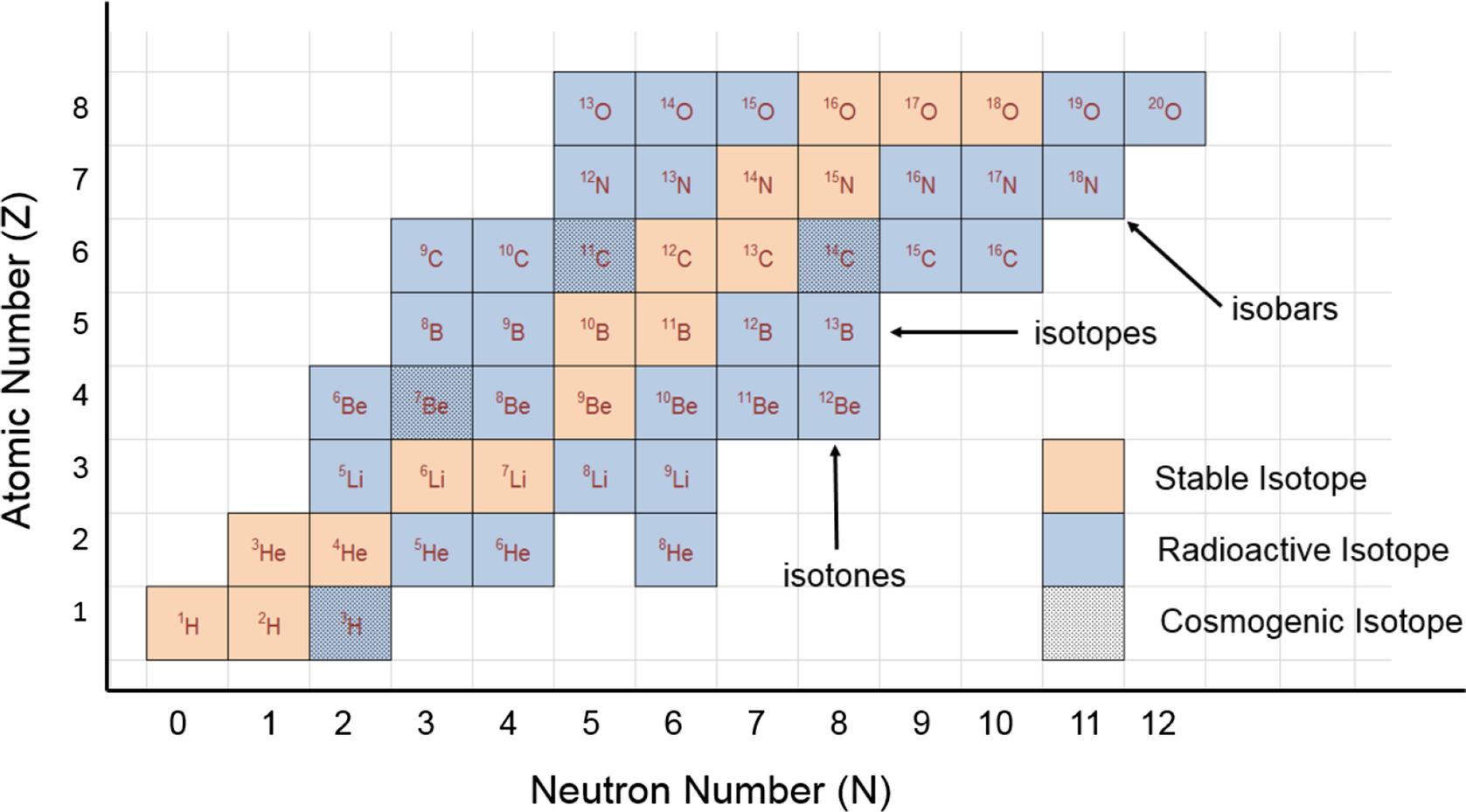
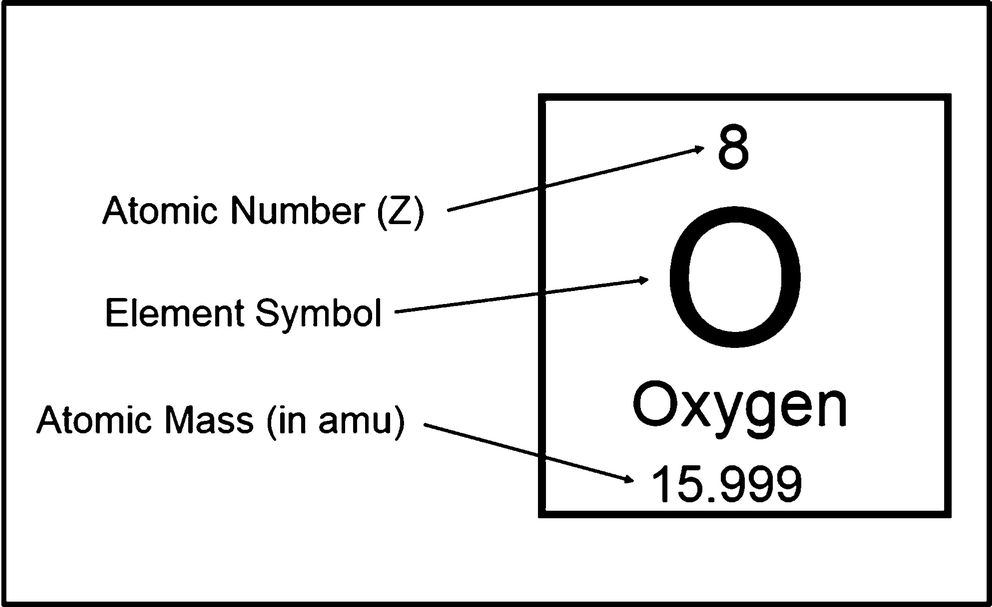
A Which corner of the nuclide symbol contains the mass number. Element C With atomic number 14 6 C With neutron number 14 6 C 8. Each chemical element has a different number of protons often with different numbers of neutrons.
Updated April 16 2018. Atomic mass of C is 12011 amu which we see in. For example nitrogen has 7 protons and 7 neutrons in its nucleus giving it a mass number of 14.
The mass number A also called atomic mass number or nucleon number is the total number of protons and neutrons together known as nucleons in an atomic nucleus. The small contribution of mass from electrons is disregarded in calculating the mass number. 1 See answer tomlinson327 is waiting for your help.
Mass number represents the mass of one particular isotope and it is a whole number for example Mass number is 13 and atomic number is 6 for the carbon isotope C-13. This is the total number of protons and neutrons in an atomic nucleus. This loss of units results from the use of a scaling ratio with respect to a carbon-12 standard and the word relative in the term relative isotopic mass refers to this scaling relative to carbon-12.
Why is this number called a mass number. The mass number is different for each different isotope of a chemical element. Fill in the table for Atom I and Atom II shown to the right.
Mass number is often denoted using a capital letter A. Atomic number in German is Atomzahl so the Z symbol for atomic number probably comes from Zahl number. Read Articles related to why is the number called a mass number.
The mass number of an element is named as such because it gives the mass of the total number of protons and neutrons in an element. D N Z A 2ZNeutron number. This can also be called the nucleon number.
B Why is it called a mass number. It is a whole number. The difference between the neutron number and the atomic number is known as the neutron excess.
Both protons and neutrons weigh approximately 1 atomic mass unit the approximately is a complication from the mass deficit. Z N A. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements.
Different isotopes of the same element have different mass numbers because their nuclei contain different numbers of neutrons. The mass number A of an atom within physics and chemistry is the total number of nucleons protons and neutrons in its nucleus. See full answer below.
The mass number is also called the nucleon number which is a better name as it describes what the number is - the number of nucleons in the nucleus. The Atomic Mass units of an element are never simple numbers because it is impossible to determine the number of molecules in one element and as. The total number of protons and neutrons in the nucleus of an atom.
The mass of a nucleus if quoted to sufficient precision in kg or in atomic mass units u will not be an integer other than in the case of 12-C where the definition of the atomic mass unit ensures this is exactly 12.

What Is An Atomic Number Definition And Examples

Atomic Number Atomic Mass And Isotopes Article Khan Academy

Atomic Number Chemistry For Non Majors
Difference Between Atomic Number And Mass Number Definition Explanation With Examples

Mass Number Versus Atomic Number And Atomic Mass
Atomic Number Mass Number And Isotopes Springerlink
Difference Between Atomic Number And Mass Number Definition Explanation With Examples
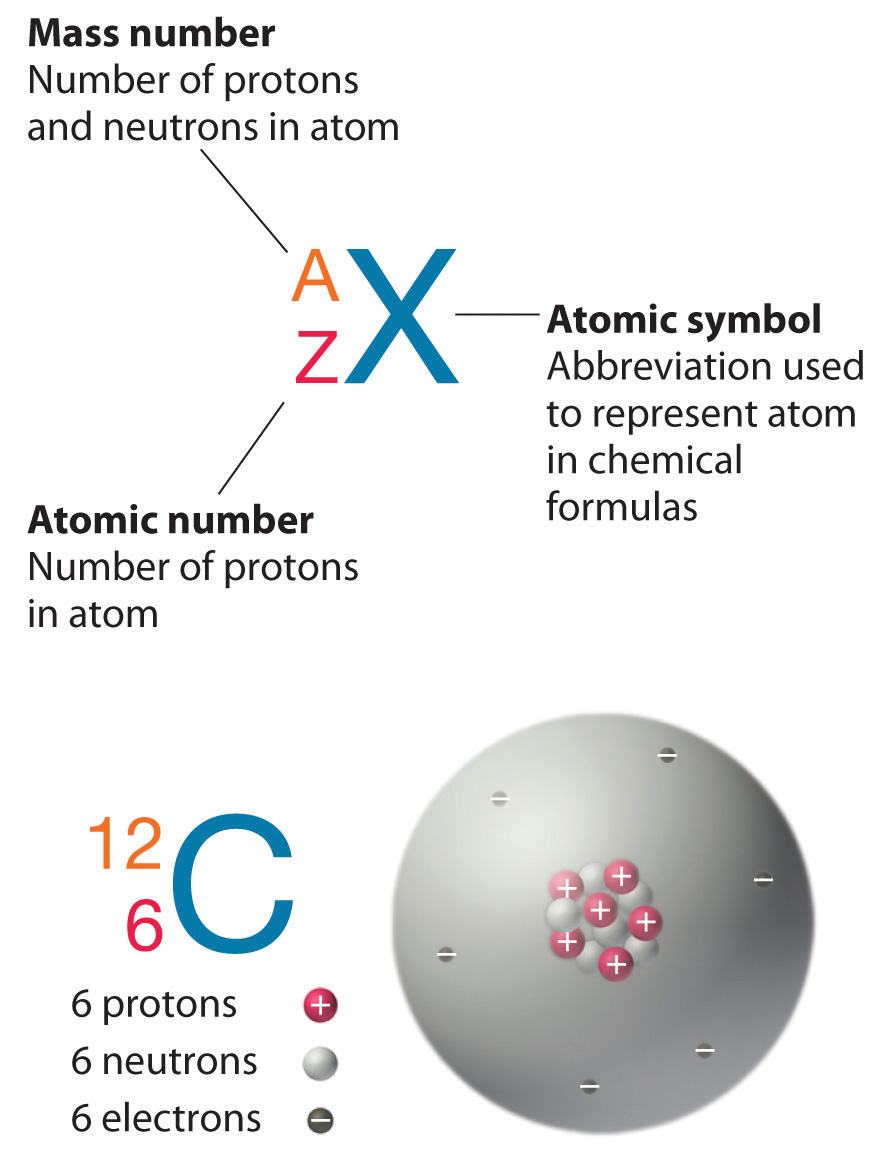
2 2 Atomic Number Mass Number And Atomic Mass Unit Chemistry Libretexts

Mass Number Definition Notation Formula Chemistrygod
Atomic Number Mass Number And Isotopes Springerlink
Atomic Number Mass Number And Isotopes Video Khan Academy
Difference Between Atomic Number And Mass Number Definition Explanation With Examples

Mass Number Definition Notation Formula Chemistrygod
5 8 Isotopes When The Number Of Neutrons Varies Chemistry Libretexts

Americium Chemical Element Britannica
Atomic Number Mass Number Definition Facts Videos Calculations With Examples And Faqs
Atomic Number Mass Number Definition Facts Videos Calculations With Examples And Faqs

All About The Periodic Table Vanderbilt Student Volunteers For Science

Atomic Number Mass Number And Isotopes Ppt Download
